Taro RX product details
Product Information
-
DIN:02310503
-
Active Ingredients :ramipril
-
Innovator Name:Altace®
-
Category:Angiotensin Converting Enzyme Inhibitor
-
Dosage form:Capsule
-
Strength:1.25mg
- Package size:
30 - UPC Code:
876515008790 -
Non-medicinal ingredients :pre-gelatinized starch and pregelatinized starch (Lycatab). Capsule shell composition: gelatin, methyl paraben, propyl paraben, iron oxide yellow and titanium dioxide
All brand names and trademarks referenced remain the property of their respective owners.
To view the available Product Monographs for Taro Pharmaceuticals Inc. and TaroPharma products please click here to access the Health Canada Drug Product Database and search by DIN or other listed criteria. You may also contact us to request the information.
For Provincial Formulary Status click here
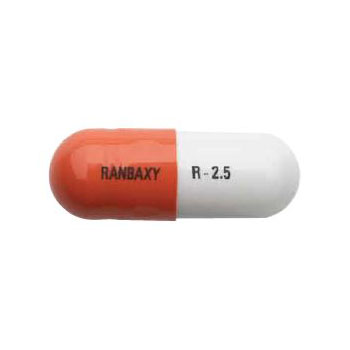
Product Information
-
DIN:02310511
-
Active Ingredients :ramipril
-
Innovator Name:Altace®
-
Category:Angiotensin Converting Enzyme Inhibitor
-
Dosage form:Capsule
-
Strength:2.5mg
- Package size:
100 Bottle 500 Bottle - UPC Code:
876515008752 876515008851 -
Non-medicinal ingredients :pre-gelatinized starch and pregelatinized starch (Lycatab). Capsule shell composition: gelatin, methyl paraben, propyl paraben, carmoisine, ponceau 4R, sunset yellow and titanium dioxide
All brand names and trademarks referenced remain the property of their respective owners.
To view the available Product Monographs for Taro Pharmaceuticals Inc. and TaroPharma products please click here to access the Health Canada Drug Product Database and search by DIN or other listed criteria. You may also contact us to request the information.
For Provincial Formulary Status click here
Product Information
-
DIN:02310538
-
Active Ingredients :ramipril
-
Innovator Name:Altace®
-
Category:Angiotensin Converting Enzyme Inhibitor
-
Dosage form:Capsule
-
Strength:5mg
- Package size:
100 Bottle 500 Bottle - UPC Code:
876515008769 876515008868 -
Non-medicinal ingredients :FD&C Blue 1, FD&C Red 40, gelatin, sodium lauryl sulfate and titanium dioxide. Capsule Imprinting Ink Composition: Black Iron Oxide, Potassium Hydroxide, Propylene Glycol, and Shellac
All brand names and trademarks referenced remain the property of their respective owners.
To view the available Product Monographs for Taro Pharmaceuticals Inc. and TaroPharma products please click here to access the Health Canada Drug Product Database and search by DIN or other listed criteria. You may also contact us to request the information.
For Provincial Formulary Status click here
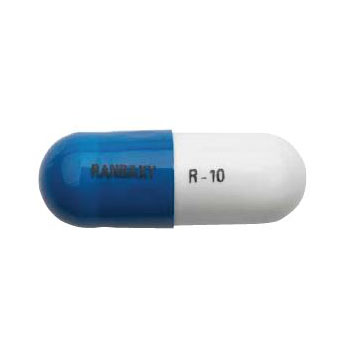
Product Information
-
DIN:02310546
-
Active Ingredients :ramipril
-
Innovator Name:Altace®
-
Category:Angiotensin Converting Enzyme Inhibitor
-
Dosage form:Capsule
-
Strength:10mg
- Package size:
100 Bottle 500 Bottle - UPC Code:
876515008776 876515008875 -
Non-medicinal ingredients :D&C Yellow 10, FD&C Blue 2, FD&C Red 3, gelatin, sodium lauryl sulfate and titanium dioxide. Capsule Imprinting Ink Composition: Black Iron Oxide, Potassium Hydroxide, Propylene Glycol, and Shellac
All brand names and trademarks referenced remain the property of their respective owners.
To view the available Product Monographs for Taro Pharmaceuticals Inc. and TaroPharma products please click here to access the Health Canada Drug Product Database and search by DIN or other listed criteria. You may also contact us to request the information.
For Provincial Formulary Status click here




 Taro Corporate
Taro Corporate Taro U.S.A
Taro U.S.A Taro Canada
Taro Canada Taro Israel
Taro Israel Sun Pharma
Sun Pharma Chattem Chemicals Inc.
Chattem Chemicals Inc.