TaroPharma RX Product Details
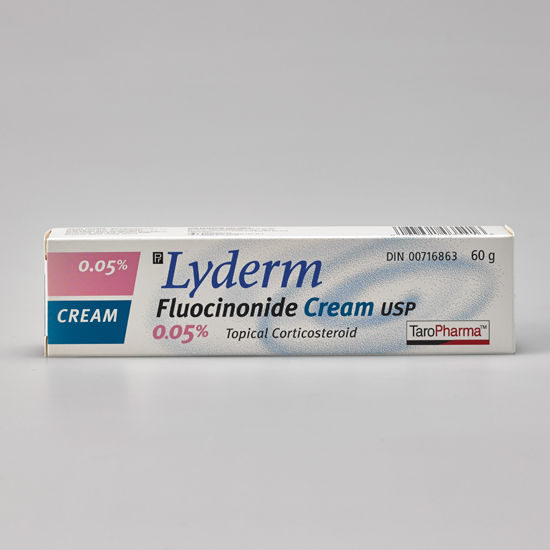
LYDERM® Cream
Product Information
-
DIN:00716863
-
Brand Reference:Lidex®
-
Active Ingredients :fluocinonide
-
Category:High Potency Topical Corticosteroid
-
Dosage form:Cream
-
Strength:0.05%
-
Route of Administration:Topical
- Package size:
60 g - UPC Code:
063691035416 -
Non-medicinal ingredients :citric acid, glycerin, 1,2,6-hexanetriol, PEG-8000, propylene glycol and stearyl alcohol
LYDERM® Cream
All brand names and trademarks referenced remain the property of their respective owners.
To view the available Product Monographs for Taro Pharmaceuticals Inc. and TaroPharma products please click here to access the Health Canada Drug Product Database and search by DIN or other listed criteria. You may also contact us to request the information.
For Provincial Formulary Status click here



 Taro Corporate
Taro Corporate Taro U.S.A
Taro U.S.A Taro Canada
Taro Canada Taro Israel
Taro Israel Sun Pharma
Sun Pharma Chattem Chemicals Inc.
Chattem Chemicals Inc.